Chronic pancreatitis
Chronic pancreatitis is the most common cause of PEI – 80 to 90% of these patients will have some degree of PEI.1 In patients with chronic pancreatitis and severe PEI, digestive lipase output has been shown to be about 2.5 IU/min – less than 0.2% of normal (3,000 IU/min).1 In order to avoid steatorrhoea, lipase secretion of greater than 5 to 10% of normal is needed, precipitating the need for effective Pancreatic Enzyme Replacement Therapy (PERT).1
Creon® has been shown to improve symptoms associated with PEI
Creon® improved the following symptoms at 1 week and at 52 weeks versus baseline:2,3
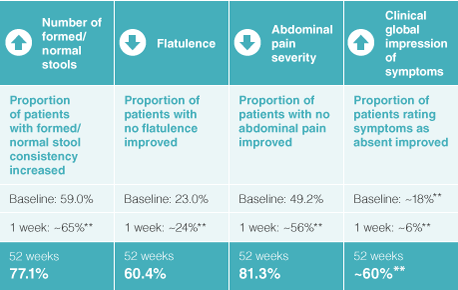
Adapted from Thorat V et al. Aliment Pharmacol Ther 2012; Ramesh H. et al. Pancreatology 2013.
** Values are estimations taken from graphs within the published paper as exact values were unpublished.
There were also significant improvements at 52 weeks in coefficients of fat absorption (CFA) and nitrogen absorption (CNA) versus baseline:3

**Values are estimations taken from graphs within the published paper as exact values unpublished.
Creon® has been shown to significantly improve patients’ nutritional status3
Several nutritional parameters significantly improved from baseline in patients with chronic pancreatitis.
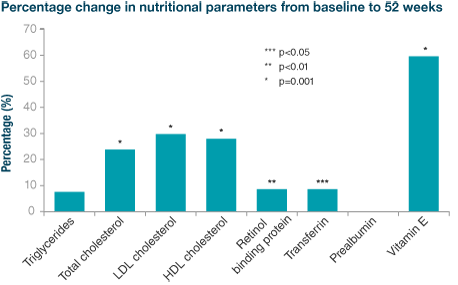
Adapted from Ramesh H. et al. Pancreatology 2013
Dose of 80,000 lipase units/meal; 40,000 lipase units/snack; 6-9 capsules per day.
Creon® has been shown to improve quality of life as measured by the SF-36 Health Survey questionnaire3
At 52 weeks, patient quality of life had improved from baseline with statistically significant changes in:
- bodily pain
- general health
- vitality
- emotional well-being
- mental health
- mental component summary.
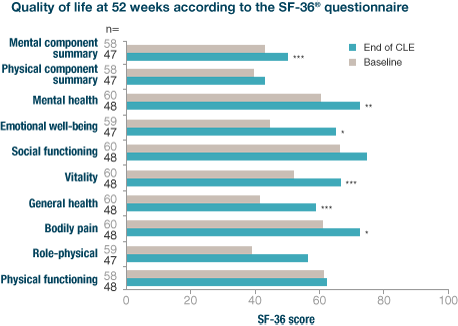
***p=0.001 vs. baseline; **p<0.01 v. baseline; *p<0.05 vs. baseline.
Adapted from Ramesh H et al. Pancreatology 2013
OLE: Open-label extension – 52 weeks.
References
- Keller J, et al. Human pancreatic exocrine response to nutrients in health and disease. Int J Pancreatol. 2005; 54(Suppl 6): 1-28
- Thorat V, et al. Randomised clinical trial: the efficacy and safety of pancreatin enteric-coated minimicrospheres (Creon 40000 MMS) in patients with pancreatic exocrine insufficiency due to chronic pancreatitis--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2012; 36(5): 426-436
- Ramesh H, et al. A 51-week, open-label clinical trial in India to assess the efficacy and safety of pancreatin 40000 enteric-coated minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. Pancreatology. 2013; 13(2): 133-139
- Imrie CW, et al. Review article: enzyme supplementation in cystic fibrosis, chronic pancreatitis, pancreatic and periampullary cancer. Aliment Pharmacol Ther. 2010; 1:1-25.
Viatris Connect is an online platform for UK healthcare professionals.
Across the website you will find news, blogs and product information.
Register to Viatris Connect today
Please note that the website contains promotional and non-promotional material including educational content and resources to help you and your patients.
REGISTER NOW
 READ NOW
READ NOW

