Pancreatic cancer
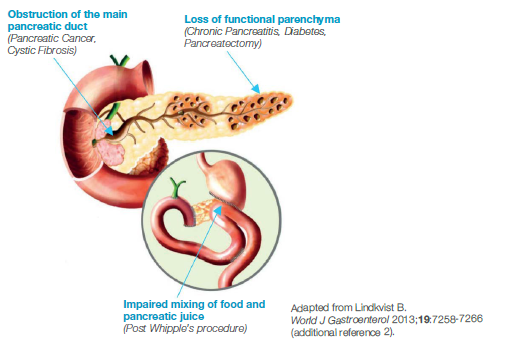
The prevalence of PEI in patients with pancreatic cancer is 80–90%, and both the site and degree of ductal obstruction will determine the severity of PEI in these patients.1 In pancreatic cancer, pancreatic enzyme insufficiency commonly occurs through either obstruction of the pancreatic duct by tumours of the head of the pancreas or through the loss of functioning pancreatic parenchyma by progressive destruction with tumour growth. Loss of pancreatic tissue following surgical procedures also contributes to pancreatic enzyme insufficiency.1-3
How PEI occurs in pancreatic cancer
Undertreated PEI impacts both quality of life and outcomes in pancreatic cancer
The resulting malabsorption is associated with bodyweight loss and malnutrition, which are in turn associated with a poor prognosis, increased morbidity and delayed recovery following surgery, significant reduction in quality of life and even interruption of cancer therapy.2
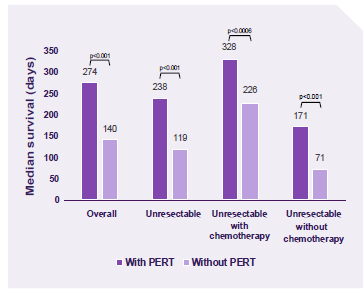
A retrospective case-controlled study demonstrated PERT usage was associated with improved survival. The results remained significant with or without chemotherapy.4
Malabsorption and weight loss in these patients are often overlooked. Most patients with cancer of the pancreatic head region are already exocrine insufficient at diagnosis. Given this high prevalence, physicians should direct their focus to diagnose and treat exocrine insufficiency with pancreatic enzymes to optimize the patient’s nutritional status and physical condition, maintain weight, endure surgery, improve tolerance to palliative chemotherapy and/or radiotherapy and improve their quality of life.5,6
NICE Guideline Recommendations for the management of PEI in pancreatic cancer
- Offer enteric-coated pancreatin for people with unresectable pancreatic cancer7
- Consider enteric-coated pancreatin before and after pancreatic cancer resection7
PEI is currently undertreated in pancreatic cancer
In 2021 the RICOCHET study, a UK prospective audit of pancreatic cancer care was published.
Patients diagnosed with pancreatic cancer at any of the hospitals in the RICOCHET study were included in the analysis for this study.
Data were collected over a 16-week period from April to July 2018, with patients followed-up for 90 days from presentation.
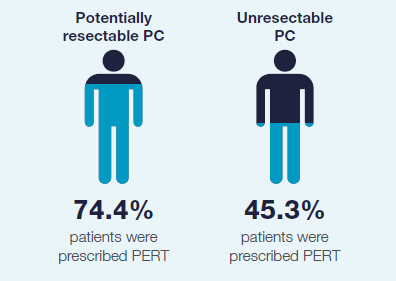
Despite NICE guidelines being in place since 2018, the RICOCHET data showed that there was underprescribing of PERT to treat PEI in patients with pancreatic cancer.8
Pancreatic enzyme supplementation should be considered in most patients with pancreatic cancer and used whenever there is clinical suspicion of exocrine insufficiency.2
Patients with pancreatic cancer struggle with symptoms of malabsorption unaware that they could be helped with medication, with a negative impact on both their physical and psychological health.9
References
- Keller J, et al. Human pancreatic exocrine response to nutrients in health and disease. Int J Pancreatol. 2005; 54(Suppl 6): 1-28
- Imrie CW, et al. Review article: enzyme supplementation in cystic fibrosis, chronic pancreatitis, pancreatic and periampullary cancer. Aliment Pharmacol Ther. 2010; 1:1-25.
- Friess H, et al. Enzyme Treatment after Gastrointestinal Surgery. Digestion. 1993; 54(Suppl 2): 48-53
- Roberts K, et al. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology. 2019; 19(1): 114-121
- Sikkens ECM, et al. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol. 2014; 48(5): e43-e46
- Guidelines for the management of patients with pancreatic, periampullary and ampullary carcinomas. Gut 2005; 54(suppl 5): v1–v16
- National institute for health and care excellence (NICE), UK. Guideline NG85. Pancreatic cancer in adults: diagnosis and management. Feb 2018.
- RICOCHET Study Group on behalf of the West Midlands Research Collaborative. Pancreatic enzyme replacement therapy in patients with pancreatic cancer: A national prospective study. Pancreatology 2021;S1424-3903(21)00469-5.
- Gooden H M and White K J. Pancreatic cancer and supportive care - pancreatic exocrine insufficiency negatively impacts on quality of life. Support Care Cancer. 2013; 21(7): 1835-1841
Viatris Connect is an online platform for UK healthcare professionals.
Across the website you will find news, blogs and product information.
Register to Viatris Connect today
Please note that the website contains promotional and non-promotional material including educational content and resources to help you and your patients.
REGISTER NOW READ NOW
READ NOW

